Using an innovative microscopy method, scientists at The Picower Institute for Learning and Memory at MIT observed how newborn neurons struggle to reach their proper places in advanced human brain tissue models of Rett syndrome, producing new insight into how developmental deficits observed in the brains of patients with the devastating disorder may emerge.
Rett syndrome, which is characterized by symptoms including severe intellectual disability and impaired social behavior, is caused by mutations in the gene MECP2. To gain new insight into how the mutation affects the early stages of human brain development, researchers in the lab of Mriganka Sur, Newton Professor of Neuroscience in MIT’s Department of Brain and Cognitive Sciences, grew 3D cell cultures called cerebral organoids, or minibrains, using cells from people with MECP2 mutations, and compared them to otherwise-identical cultures without the mutations. Then the team led by postdoc Murat Yildirim examined the development of each type of minibrain using an advanced imaging technology called third harmonic generation (THG) three-photon microscopy.
THG, which Yildirim has helped to pioneer in Sur’s lab working with MIT mechanical engineering Professor Peter So, allows for very high-resolution imaging deep into live, intact tissues without having to add any chemicals to label cells. The new study, published in eLife, is the first to use THG to image organoids, leaving them virtually undisturbed, Yildirim said. Previous organoid imaging studies have required using technologies that cannot image all the way through the 3D tissue, or methods that require killing the cultures: either slicing them into thin sections or chemically clearing and labeling them.
Three-photon microscopy employs a laser, but Yildirim and So custom engineered the lab’s microscope to apply no more power to the tissue than a cat toy laser pointer (less than 5 milliwatts).
“You should make sure you are not changing or affecting the neuronal physiology in any adverse way,” Yildirim says. “You should really keep everything intact and make sure you are not bringing something external that could be damaging. That’s why we are so careful about power (and chemical labeling).”
Even at low power, they achieved adequate signal to achieve label-free, intact imaging of fixed and live organoids. To validate that they compared their THG images to images made via more traditional chemical labeling methods.
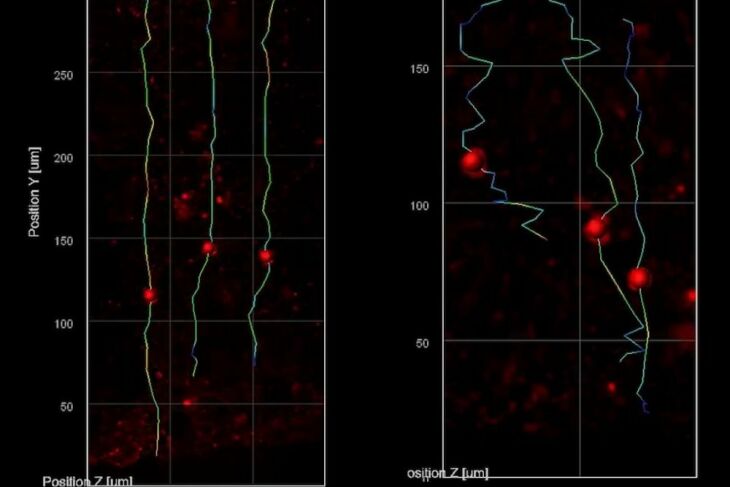
The THG system allowed them to track the migration of newborn neurons as they made their way from the rim around open spaces in the minibrains (called ventricles) to the outer edge, which is directly analogous to the brain’s cortex. They saw that the nascent neurons in the minibrains modeling Rett syndrome moved slowly and in meandering paths compared to the faster motion in straighter lines exhibited by the same cell types in minibrains without MECP2 mutation. Sur says the consequences of such migration deficits are consistent with what scientists, including in his lab, have hypothesized is going on in fetuses with Rett syndrome.
“We know from postmortem brains and brain imaging methods that things go awry during brain development in Rett syndrome, but it has been astonishingly difficult to figure out what and why,” says Sur, who directs the Simons Center for the Social Brain at MIT. “This method has enabled us to directly visualize a key contributor.” THG images tissues without labels because it is very sensitive to changes in the refractive index of materials, Yildirim says. It therefore resolves boundaries between biological structures, such as blood vessels, cell membranes, and extracellular spaces. Because neural shapes change during their development, the team was able to also clearly see the delineation between the ventricular zone (the area around the ventricles where the newborn neurons emerge) and the cortical plate (an area that mature neurons settle into). It was also very easy to resolve various ventricles and segment them into distinct regions.